

2020-09-25
On September 25, 2020, BRL Medicine Inc., a company specialized in gene therapy and cell drug R&D, announced for the first time that clinical trials of non-viral PD1 specific targeted CART therapy in relapsed/refractory B-cell non-Hodgkin lymphoma, undertaken by the company in cooperation with East China Normal University and the First Affiliated Hospital, School of Medicine, Zhejiang University, has achieved significant breakthroughs. This is the world’s first application of gene editing technology to realize PD1-knockin CAR-T treatment, and is also the world’s first clinical trial treating lymphoma with non-viral PD1 specific targeted CAR-T cells.
The latest research results, finished by East China Normal University, the First Affiliated Hospital, School of Medicine, Zhejiang University and BRL Medicine, were announced on the preprint platform medRxiv on September 24, 2020.
PD1 specific targeted CD19-CART is BRL Medicine’s Quikin CAR-T Platform technology using its proprietary IP, precisely inserting CAR cassette into PD1 locus without using virus, thus generating the CAR-T product in just one step. The product combines PD1 immune checkpoint inhibition with CAR-T anti-tumor activity, generating effects of both anti-PD1 immunotherapy and CAR-T therapy. Several ongoing clinical trials demonstrate the outstanding safety and effectiveness of this CAR-T product.
Two patients showed complete remission following a three-month treatment
The clinical trial program enrolled 15 patients, among which, four patients who were able to be evaluated showed partial remission (PR) after receiving the one-month treatment, while two patients showed complete remission (CR) after three months of treatment.
There were no CAR-T cell related high-grade (≥3) adverse events?during the entire treatment, including cytokine release syndrome (CRS)?and neurologic toxicity. After infusion, the CAR-T cells were well sustained in vivo.
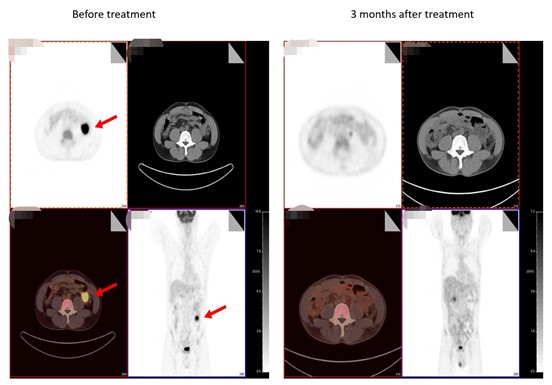
△ Comparison of pre- and post-treatment imaging
The Quikin CAR-T technology?produces CAR-T cells without using virus, greatly reducing high costs of producing CAR-T products, while avoiding the cancer risk from random insertion. The regulation of T cell endogenous genes and the constant expression of CAR are realized in just one step. Compared with other CAR-T technologies, Quikin CAR-T has a variety of advantages, including a simpler process, fewer production links, shorter time preparation and higher product uniformity.
Liu Mingyao, chief scientist at Bioraylab and professor at East China Normal University, said, “Without using virus, the Quikin CAR-T technology can realize the precise integration of CAR cassette into genome as well as regulation of T cell endogenous genes in just one step, delivering many unparalleled advantages compared with existing CAR-T technologies. The technology offers a strong platform for the development of more diverse CAR-T products in the future.”
Zhang Jiqin, the main person in charge of the project, the first author of the paper, the R&D director of Bioraylab Bio-Innovation CAR-T, and the associate researcher of the School of Life Sciences of ECNU, said: "In recent years, we realized that the use of gene editing technology to prepare non-viral targeted integration of CAR-T cells is a very promising direction. Through a large number of methods to try and After exploring the conditions, we have established a mature Quikin CART? technology platform. This technology does not require the use of any virus, and can produce CAR-T cells in one step. It has many advantages that cannot be compared with the existing CAR-T technology. We look forward to using this technology platform in the future. It is possible to develop more successful CAR-T products and achieve greater breakthroughs in clinical treatment."
It is reported that in addition to the ongoing non-viral PD1 targeted integration of CD19-CART clinical research, Bioraylab Biosciences is also deploying other non-viral products for solid tumors. The virus integrates CAR-T products at designated points in order to achieve greater breakthroughs in CAR-T therapy.