- Global | English
- Search
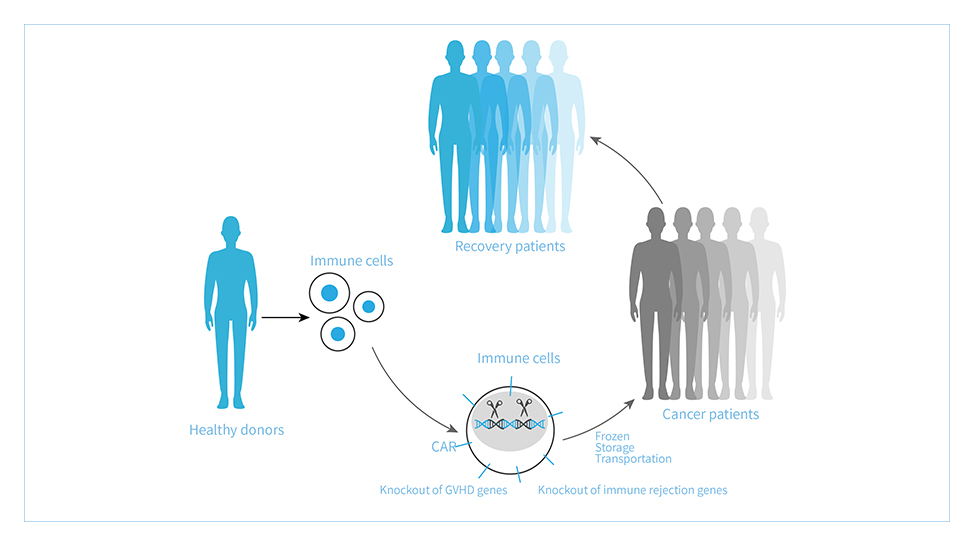
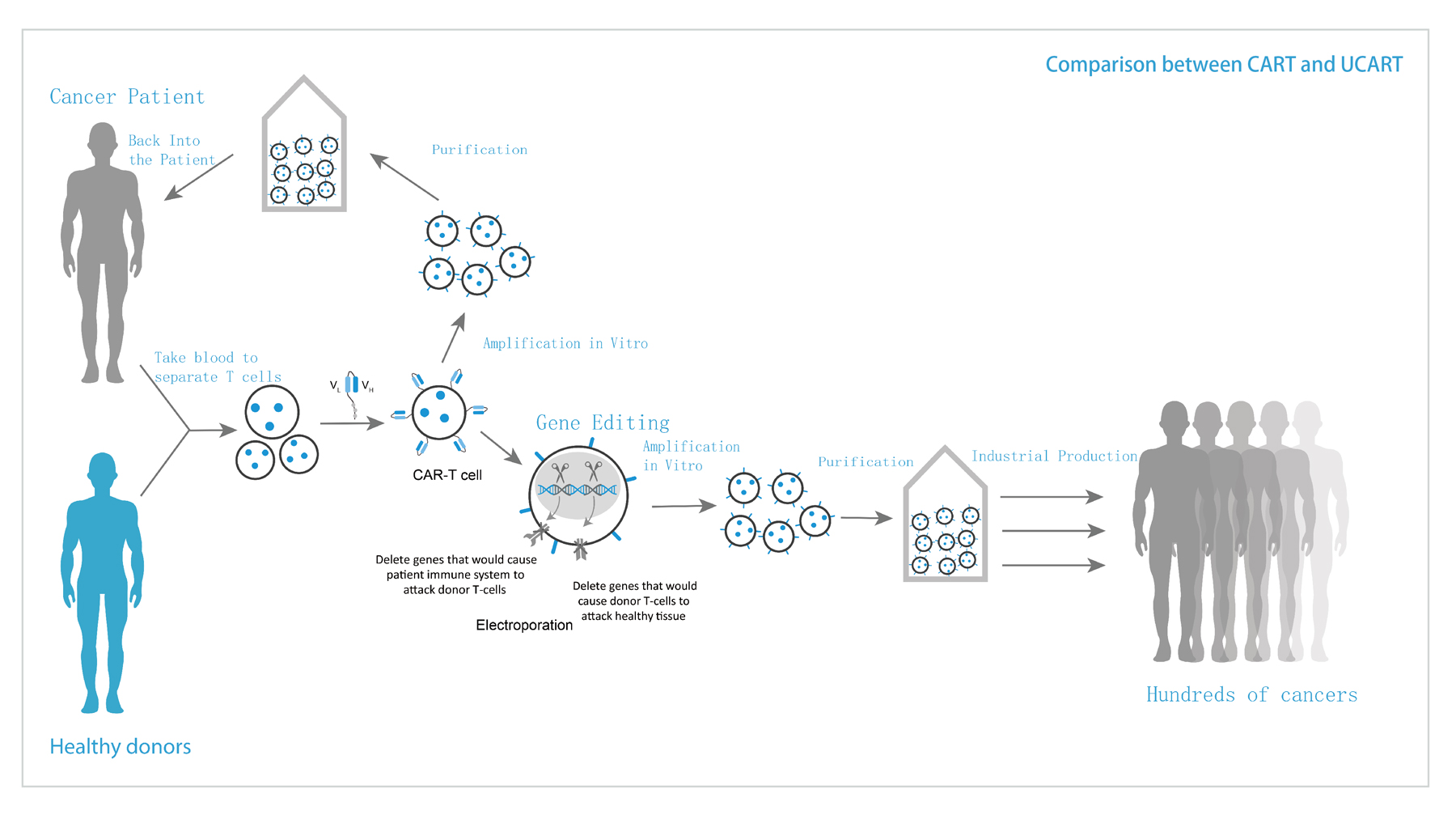
Universal Cell Platform(TyUCell?)refers to the use of gene editing technology to transform allogeneic immune cells to eliminate immune rejection.
lEARN MORE TyUCell?The Quikin CAR-T Platform(Quikin CART) generates T cell products with gene knockout and stable integration of CAR cassette in one step through CRISPR gene editing technology.
lEARN MORE Quikin CARTEnhanced T cell Platforms(HyperTCell?), including HyperCART ? and HyperTCRT , are mainly to solve the global highlighted problem in solid tumor therapy through genetic modification of T cells.
lEARN MORE HyperTCell?
Universal Cell Platform (TyUCell?) refers to the use of gene editing technology to transform allogeneic immune cells to eliminate immune rejection. On the basis of ensuring the safety and effectiveness of cell products, it realizes the generalization of immune cell therapy products. Tyucell cell products have the advantages of low cost, wide application range, short production cycle, stable preparation process and good therapeutic effect. The platform can be used for clinical treatment after a large number of expansion of cells from healthy donors. At the same time, it can realize the large-scale and batch production of cell products, significantly reduce the production cost, shorten the waiting time of patients, and provide modern cell therapy products that meet the urgent needs of clinic.